
Back تفاعل غرينيار Arabic Grignardova reakce Czech Grignardreaktion Danish Grignard-Reaktion German Reacción de Grignard Spanish Grignardi reaktsioon Estonian Grignarden erreakzio Basque واکنش گرینیارد Persian Réaction de Grignard French Գրինյարի ռեակցիա Armenian
| Classical Grignard reaction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Named after | Victor Grignard | ||||||||||
| Reaction type | Coupling reaction | ||||||||||
| Reaction | |||||||||||
| |||||||||||
| Identifiers | |||||||||||
| Organic Chemistry Portal | grignard-reaction | ||||||||||
| RSC ontology ID | RXNO:0000014 | ||||||||||

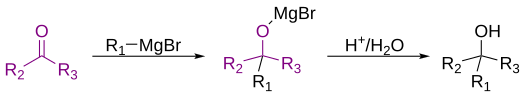
The Grignard reaction (French: [ɡʁiɲaʁ]) is an organometallic chemical reaction in which, according to the classical definition, carbon alkyl, allyl, vinyl, or aryl magnesium halides (Grignard reagent) are added to the carbonyl groups of either an aldehyde or ketone under anhydrous conditions.[1][2][3] This reaction is important for the formation of carbon–carbon bonds.[4][5]
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Chapter 19: Carboxylic Acids. Organic Chemistry 4e Carey. mhhe.com
- ^ Cite error: The named reference
:12was invoked but never defined (see the help page). - ^ Shirley, D. A. (1954). "The Synthesis of Ketones from Acid Halides and Organometallic Compounds of Magnesium, Zinc, and Cadmium". Org. React. 8: 28–58.
- ^ Huryn, D. M. (1991). "Carbanions of Alkali and Alkaline Earth Cations: (ii) Selectivity of Carbonyl Addition Reactions". In Trost, B. M.; Fleming, I. (eds.). Comprehensive Organic Synthesis, Volume 1: Additions to C—X π-Bonds, Part 1. Elsevier Science. pp. 49–75. doi:10.1016/B978-0-08-052349-1.00002-0. ISBN 978-0-08-052349-1.